Ammonium hexafluorophosphate is an inorganic salt with the chemical formula NH4PF6. It consists of an ammonium cation (NH4+) paired with a hexafluorophosphate anion (PF6−), forming a colorless, crystalline solid under standard conditions. The PF6− anion is highly symmetrical, with octahedral geometry around the phosphorus atom, and is chemically stable due to the strong P–F bonds. This stability, combined with the ionic nature of the compound, makes ammonium hexafluorophosphate soluble in polar solvents such as water, acetonitrile, and methanol, while being largely inert toward weak acids and bases.
The development of ammonium hexafluorophosphate is connected to the study of hexafluorophosphate salts in inorganic and organometallic chemistry. Hexafluorophosphate salts are commonly used because the PF6− anion is non-coordinating and highly resistant to hydrolysis, making it an ideal counterion for stabilizing cationic species. Ammonium hexafluorophosphate, in particular, is employed in applications where a volatile or removable cation is desired, as the ammonium ion can be decomposed thermally or displaced in chemical reactions, leaving the PF6− anion to stabilize reactive intermediates.
The compound is synthesized by neutralization of hexafluorophosphoric acid (HPF6) with an ammonium base such as ammonium hydroxide. This reaction forms NH4PF6 as a crystalline solid, which can be purified by recrystallization from polar solvents. The procedure is straightforward due to the high solubility of the salt and the stability of the PF6− anion. Careful control of reaction stoichiometry and temperature is necessary to avoid the formation of acidic by-products or decomposition of the hexafluorophosphate.
Ammonium hexafluorophosphate is primarily used in inorganic and organometallic chemistry as a source of the PF6− counterion. Its non-coordinating nature allows it to stabilize cationic transition metal complexes, organic cations, and ionic liquids without interfering with the metal-ligand or cation-anion interactions. This property is particularly valuable in electrochemistry, where PF6− salts are used as electrolytes for non-aqueous solutions, providing high conductivity and stability over a wide electrochemical window.
Chemically, the PF6− anion in ammonium hexafluorophosphate is highly resistant to nucleophilic attack and hydrolysis, allowing it to remain intact under conditions that would decompose other anions. The ammonium cation, on the other hand, is labile and can participate in acid-base reactions or be replaced by other cations in metathesis reactions. This duality allows the compound to serve as both a stabilizing salt and a precursor to other hexafluorophosphate salts, such as those containing alkali metals, transition metals, or organic cations.
In materials science, ammonium hexafluorophosphate is used in the preparation of ionic liquids and as a component in electrochemical devices, including batteries, capacitors, and sensors. Its high solubility and stability make it suitable for use in both aqueous and non-aqueous electrolytes. In organic synthesis, it is sometimes employed as a mild source of PF6− to generate stable cationic intermediates that can participate in selective reactions without unwanted side reactions from coordinating anions.
From a safety perspective, ammonium hexafluorophosphate should be handled with care. It is generally stable under ambient conditions, but exposure to strong acids or bases can lead to hydrolysis, releasing corrosive hydrogen fluoride or other toxic by-products. Proper protective equipment, including gloves and eye protection, is recommended, along with adequate ventilation to avoid inhalation of dust or fumes.
Overall, ammonium hexafluorophosphate is a chemically stable, highly soluble, and non-coordinating salt that plays an important role in inorganic, organometallic, and materials chemistry. Its combination of an inert hexafluorophosphate anion and a labile ammonium cation makes it a versatile reagent for stabilizing cationic species, serving as a precursor to other salts, and providing conductivity in electrochemical applications. Its unique properties continue to support its widespread use in laboratory and industrial processes.
References
2025. Fabrication and characterization of polymer electrolyte based on PAN with NaSCN for solid-state sodium-ion batteries. Ionics.
DOI: 10.1007/s11581-025-06549-x
|

















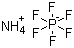
 GHS05 Danger Details
GHS05 Danger Details