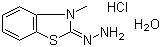
3-Methyl-2-benzothiazolinone hydrazone hydrochloride monohydrate (commonly abbreviated as MBTH hydrochloride) is an organic compound derived from 2-benzothiazolinone, in which a hydrazone functional group is attached at the 2-position and the nitrogen is protonated as a hydrochloride salt. The molecular formula is C8H10N3S·HCl·H2O. It typically appears as a red to reddish-brown crystalline solid that is soluble in water and polar organic solvents. The presence of the hydrazone moiety and the protonated hydrochloride form contributes to its chemical reactivity and stability, while the monohydrate ensures crystalline integrity and reproducibility in reactions.
The discovery of MBTH hydrochloride is associated with the development of chromogenic reagents for analytical chemistry. It was identified as a highly effective reagent for the detection and quantification of oxidizing agents, phenolic compounds, and certain enzymes. Its ability to form colored products upon oxidation makes it particularly useful in colorimetric assays, where the intensity of the color correlates with the concentration of the target analyte.
Synthesis of 3-methyl-2-benzothiazolinone hydrazone hydrochloride monohydrate generally involves condensation of 3-methyl-2-benzothiazolinone with hydrazine under acidic conditions to form the hydrazone. The resulting hydrazone is then converted into its hydrochloride salt by treatment with hydrochloric acid, and crystallization from water or ethanol produces the monohydrate form. Reaction conditions such as temperature, solvent, and reagent stoichiometry are carefully controlled to maximize yield and prevent decomposition of the hydrazone functionality.
Chemically, MBTH hydrochloride acts as a nucleophile at the hydrazone nitrogen and as an electron-rich aromatic system capable of undergoing oxidation. The compound readily reacts with oxidizing agents in the presence of a suitable catalyst or peroxidase enzyme to form a colored azo or quinoneimine derivative. This chromogenic reaction underpins its use in analytical applications. The protonated hydrochloride form improves solubility in aqueous solutions, while the monohydrate ensures consistent crystalline structure and purity for reliable assay performance.
In practical applications, MBTH hydrochloride monohydrate is widely used as a reagent for spectrophotometric and enzymatic assays. It is employed to detect phenolic compounds, amino acids, and certain enzymatic activities, such as peroxidase and oxidase. Upon oxidation, the reagent forms a highly colored product that can be quantified using spectrophotometry. This makes it valuable in environmental analysis, clinical chemistry, and food chemistry, where accurate measurement of analytes is required. Additionally, it has been used in combination with other chromogenic reagents to enhance sensitivity and specificity in analytical protocols.
Physically, the compound is stable under ambient conditions when stored in a dry, cool environment, but it should be protected from strong oxidizing agents that can prematurely convert it to colored products. Standard laboratory precautions, including the use of gloves, goggles, and protective clothing, are recommended, as the compound can cause irritation upon contact.
Overall, 3-methyl-2-benzothiazolinone hydrazone hydrochloride monohydrate is a chemically and analytically important reagent. Its combination of an electron-rich aromatic hydrazone system, protonated hydrochloride form, and crystalline monohydrate structure enables selective and sensitive chromogenic reactions. The compound continues to be a valuable tool in analytical chemistry for colorimetric and enzymatic assays, providing reliable and reproducible detection of phenolic and oxidizable substances in diverse applications.
References
2024. Lipoic Acid Can Maintain Stimulation of the Antioxidant System at Lower Reactive Oxygen Species, Ascorbate and Glutathione Levels in Osmotic Stressed Maize. Russian Journal of Plant Physiology.
DOI: 10.1134/s1021443724604373
|





















 GHS06;GHS07;GHS08 Danger Details
GHS06;GHS07;GHS08 Danger Details