Online Database of Chemicals from Around the World
| Epochem Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (21) 6760-1595 6760-1597 | |||
 |
seth_wang@epochem.com | |||
 |
QQ chat | |||
| Chemical manufacturer since 2006 | ||||
| chemBlink standard supplier since 2005 | ||||
| Danyang Dajiang Chemical Factory | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (516) 8878-8888 8878-1988 | |||
 |
dajiang@dajiang-chem.com | |||
| Chemical manufacturer | ||||
| chemBlink standard supplier since 2007 | ||||
| Simagchem Corporation | China | Inquire | ||
|---|---|---|---|---|
 |
+86 13806087780 | |||
 |
sale@simagchem.com | |||
| Chemical manufacturer since 2002 | ||||
| chemBlink standard supplier since 2008 | ||||
| Shanghai Yongzeng Chemical Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (21) 6528-1118 | |||
 |
sales@yongzchem.com yongzchem@hotmail.com | |||
 |
QQ chat | |||
| Chemical manufacturer since 2008 | ||||
| chemBlink standard supplier since 2010 | ||||
| Wilshire Technologies, Inc. | USA | Inquire | ||
|---|---|---|---|---|
 |
+1 (609) 683-1117 | |||
 |
Wilshire-info@evonik.com | |||
| Chemical manufacturer since 1997 | ||||
| chemBlink standard supplier since 2010 | ||||
| BOC Sciences | USA | Inquire | ||
|---|---|---|---|---|
 |
+1 (631) 485-4226 | |||
 |
info@bocsci.com | |||
| Chemical manufacturer | ||||
| chemBlink standard supplier since 2010 | ||||
| Intatrade Chemicals GmbH | Germany | Inquire | ||
|---|---|---|---|---|
 |
+49 (3493) 605-465 | |||
 |
sales@intatrade.de | |||
| Chemical distributor | ||||
| chemBlink standard supplier since 2011 | ||||
| Tetrahedron Scientific Inc. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (571) 8848-9473 +86 18072878644 | |||
 |
sales@thsci.com | |||
| Chemical manufacturer | ||||
| chemBlink standard supplier since 2011 | ||||
| Classification | Organic raw materials >> Heterocyclic compound |
|---|---|
| Name | 4,4'-Bipyridine |
| Synonyms | 4,4'-Dipyridyl; gamma,gamma'-Dipyridyl |
| Molecular Structure | 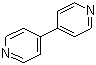 |
| Molecular Formula | C10H8N2 |
| Molecular Weight | 156.19 |
| CAS Registry Number | 553-26-4 |
| EC Number | 209-036-3 |
| SMILES | C1=CN=CC=C1C2=CC=NC=C2 |
| Density | 1.1±0.1 g/cm3 Calc.* |
|---|---|
| Melting point | 109 - 112 ºC (Expl.) |
| Boiling point | 304.8 ºC 760 mmHg (Calc.)*, 305 ºC (Expl.) |
| Flash point | 104.3±12.0 ºC (Calc.)* |
| Solubility | water: slightly soluble (Expl.) |
| Index of refraction | 1.581 (Calc.)* |
| * | Calculated using Advanced Chemistry Development (ACD/Labs) Software. |
| Hazard Symbols |


 GHS06;GHS07;GHS09 Danger Details
GHS06;GHS07;GHS09 Danger Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hazard Statements | H301+H311-H301-H311-H315-H319-H335-H411 Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Precautionary Statements | P261-P262-P264-P264+P265-P270-P271-P273-P280-P301+P316-P302+P352-P304+P340-P305+P351+P338-P316-P319-P321-P330-P332+P317-P337+P317-P361+P364-P362+P364-P391-P403+P233-P405-P501 Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hazard Classification | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Transport Information | UN 2811 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| SDS | Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||
|
4,4'-Bipyridine is an organic heteroaromatic compound consisting of two pyridine rings connected at their 4 positions. Its molecular formula is C10H8N2. It appears as a pale yellow crystalline solid that is moderately soluble in polar organic solvents such as ethanol, acetone, and dimethyl sulfoxide, while being only sparingly soluble in water. The planar structure and nitrogen atoms at the pyridine rings provide sites for coordination to metal centers, making 4,4'-bipyridine an important ligand in coordination chemistry. The discovery of 4,4'-bipyridine is linked to the exploration of polypyridine compounds in the mid-20th century. It has been widely studied for its ability to form stable complexes with transition metals, particularly in the synthesis of coordination polymers, metal-organic frameworks, and supramolecular assemblies. The two pyridine rings, connected through a single bond, allow for planarity and conjugation, which contribute to its electronic and structural properties in both neutral and cationic forms. Synthesis of 4,4'-bipyridine is typically carried out via oxidative coupling of pyridine under controlled conditions. One classical method involves the reaction of pyridine with a suitable oxidizing agent, such as sodium persulfate or iron(III) salts, which promotes the formation of the carbon-carbon bond between the 4 positions of two pyridine molecules. Alternative methods use metal-catalyzed cross-coupling reactions of halopyridines. Purification of the product is usually achieved by recrystallization from polar solvents or chromatographic techniques to remove unreacted pyridine and by-products. Chemically, 4,4'-bipyridine acts as a bidentate ligand capable of coordinating to metal centers through the nitrogen atoms in each pyridine ring. It is prone to quaternization with alkyl halides to form viologen derivatives, which are redox-active and widely used in electrochemical applications. The aromatic nitrogen atoms also allow participation in hydrogen bonding and π-π stacking interactions, which are essential in supramolecular and materials chemistry. In practical applications, 4,4'-bipyridine is a key component in the construction of coordination polymers and metal-organic frameworks due to its linear geometry and ability to bridge metal centers. It is also employed in the synthesis of viologen compounds, which serve as electron transfer mediators, redox-active materials, and herbicides. Its use in supramolecular chemistry includes the formation of molecular cages, rotaxanes, and other self-assembled architectures. From a physical standpoint, 4,4'-bipyridine is stable under normal laboratory conditions but can react with strong oxidizers and acids. It is handled with standard laboratory precautions, including gloves and eye protection, as it can cause irritation upon contact. The solid is typically stored in a dry, cool environment to prevent degradation or contamination. Overall, 4,4'-bipyridine is a versatile heteroaromatic compound with significant importance in coordination chemistry, materials science, and supramolecular design. Its linear geometry, nitrogen donor atoms, and ability to undergo quaternization make it valuable as a ligand, redox-active compound, and building block for advanced chemical architectures. The compound continues to be widely used in research and industrial applications involving metal complexes, redox chemistry, and self-assembled materials. References 2025. Synthesis, characterization, antimicrobial and antioxidant evaluation of copper(II) complexes with a leucine-derived ligand. Monatshefte f�r Chemie - Chemical Monthly. DOI: 10.1007/s00706-025-03340-6 2025. Impact of N-heteroaromatic compounds in chromium(VI) directed oxidation of organic substrates. Research on Chemical Intermediates. DOI: 10.1007/s11164-025-05678-2 |
| Market Analysis Reports |
| List of Reports Available for 4,4'-Bipyridine |