Poly(tetrafluoroethylene) (PTFE) is a high-performance fluoropolymer composed of repeating tetrafluoroethylene monomer units with the molecular formula (C2F4)n. It is a linear, semi-crystalline polymer characterized by a fully fluorinated carbon backbone, in which all hydrogen atoms of polyethylene are replaced with fluorine atoms. This structure gives PTFE exceptional chemical stability, high thermal resistance, and extremely low surface energy. PTFE appears as a white, waxy powder in its raw form or as solid sheets, rods, and films in processed materials. It is insoluble in water and most organic solvents, but it can be machined or sintered into various shapes.
The discovery of PTFE traces back to 1938 when Roy J. Plunkett at DuPont accidentally polymerized tetrafluoroethylene gas while attempting to develop new refrigerants. The resulting white, waxy polymer demonstrated remarkable chemical inertness and low friction, which quickly led to exploration of its commercial and industrial applications. PTFE was first marketed under the brand name Teflon and has since become one of the most widely used fluoropolymers worldwide.
PTFE is synthesized primarily through free-radical polymerization of tetrafluoroethylene (TFE) gas in aqueous suspension or emulsion processes. The polymerization is initiated using persulfate or other radical initiators under controlled pressure and temperature. The polymer precipitates as a fine powder, which is then washed, dried, and processed into desired forms via compression molding, extrusion, or sintering. PTFE cannot be melt-processed like conventional thermoplastics due to its extremely high melting point (~327°C) and high viscosity in the molten state.
Chemically, PTFE is exceptionally stable due to the strength of the carbon-fluorine bonds and the shielding effect of the fluorine atoms around the carbon backbone. This stability makes it highly resistant to acids, bases, oxidizers, and solvents at a wide range of temperatures. Its low coefficient of friction, non-stick properties, and electrical insulating ability result from the smooth surface and non-polar character of the polymer. PTFE is non-flammable and retains mechanical properties over a broad temperature range, from cryogenic conditions to well above 200°C.
In practical applications, PTFE is used in numerous industries due to its unique combination of chemical resistance, thermal stability, and low friction. It is widely applied as non-stick coatings for cookware, protective linings for chemical vessels, gaskets, seals, and hoses in aggressive chemical environments. PTFE films and sheets are used as dielectric layers in electronics, insulation for wiring, and as barriers in high-temperature processes. Its biocompatibility also allows medical applications, including vascular grafts, catheters, and surgical meshes. The polymer’s lubrication properties are exploited in mechanical parts, bearings, and sliding components.
Physically, PTFE is chemically inert, thermally stable, and resistant to UV degradation. It is non-adhesive, hydrophobic, and exhibits excellent dielectric properties. Handling precautions are minimal under normal conditions, but care must be taken to avoid inhalation of fine powders and to prevent thermal decomposition, which can release toxic fluorinated gases at very high temperatures.
Overall, poly(tetrafluoroethylene) is a highly versatile fluoropolymer with extraordinary chemical, thermal, and mechanical properties. Its combination of inertness, low friction, and electrical insulation makes it indispensable in industrial, chemical, electronic, and consumer applications, representing a benchmark material for high-performance polymer systems.
|



















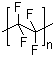
 GHS05 Details
GHS05 Details