Online Database of Chemicals from Around the World
| Alfa Chemistry | USA | Inquire | ||
|---|---|---|---|---|
 |
+1 (201) 478-8534 | |||
 |
inquiry@alfa-chemistry.com | |||
| Chemical distributor since 2012 | ||||
| chemBlink standard supplier since 2012 | ||||
| Sigma-Aldrich, Inc. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (21) 6141-5566 800-819-3336 | |||
 |
orderCN@sial.com | |||
| Chemical manufacturer since 1992 | ||||
| chemBlink standard supplier since 2013 | ||||
| Hangzhou Leap Chem Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (571) 8771-1850 | |||
 |
market19@leapchem.com | |||
 |
QQ chat | |||
| Chemical manufacturer since 2006 | ||||
| chemBlink standard supplier since 2015 | ||||
| Natural Micron Pharm Tech Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (0538) 535-2278 | |||
 |
info@nmpharmtech.com | |||
| Chemical manufacturer since 2012 | ||||
| chemBlink standard supplier since 2025 | ||||
| Classification | Analytical chemistry >> Standard >> Forensic and veterinary standards |
|---|---|
| Name | N1-Methylnicotinamide chloride |
| Synonyms | 1-methylpyridin-1-ium-3-carboxamide chloride |
| Molecular Structure | 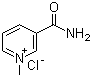 |
| Molecular Formula | C7H9N2O.Cl |
| Molecular Weight | 172.61 |
| CAS Registry Number | 1005-24-9 |
| EC Number | 686-866-6 |
| SMILES | C[N+]1=CC=CC(=C1)C(=O)N.[Cl-] |
| Melting point | 207-209 ºC (water acetone )* |
|---|---|
| * | Kosower, Edward M.; Journal of the American Chemical Society 1956, V78, P3493-7. |
| Hazard Symbols |
 GHS07 Warning Details
GHS07 Warning Details | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hazard Statements | H315-H319 Details | ||||||||||||||||
| Precautionary Statements | P264-P264+P265-P280-P302+P352-P305+P351+P338-P321-P332+P317-P337+P317-P362+P364 Details | ||||||||||||||||
| Hazard Classification | |||||||||||||||||
| |||||||||||||||||
| SDS | Available | ||||||||||||||||
|
N1-Methylnicotinamide chloride is a quaternary ammonium salt derived from nicotinamide, itself a biologically important form of vitamin B3. The compound consists of a nicotinamide core in which the ring nitrogen is methylated, forming a positively charged pyridinium species that is paired with chloride as the counterion. Its scientific relevance is closely connected to studies of nicotinamide metabolism, redox chemistry, and the broader biological roles of nicotinamide-derived cofactors. The discovery of N1-methylnicotinamide chloride is rooted in early twentieth-century research on vitamins and their metabolic fate. As niacin and nicotinamide were identified as essential nutrients preventing pellagra, researchers began to investigate how these compounds were transformed in the body. Methylated metabolites of nicotinamide were detected in biological fluids during studies of vitamin B3 metabolism, leading to the identification of N1-methylnicotinamide as a principal end product of nicotinamide methylation. The isolation and characterization of its chloride salt allowed chemists to study this metabolite in a stable and well-defined form. From a chemical standpoint, N1-methylnicotinamide chloride exemplifies the reactivity and properties of pyridinium salts. The quaternization of the nicotinamide ring nitrogen alters the electronic structure of the molecule, increasing its polarity and water solubility. This modification also changes its redox behavior compared with nicotinamide and related compounds such as nicotinamide adenine dinucleotide. These features have made N1-methylnicotinamide chloride useful as a model compound in studies exploring the chemistry of pyridinium ions and their interactions in aqueous environments. The biological significance of N1-methylnicotinamide chloride is primarily linked to its role as a metabolic product rather than as a nutrient itself. In mammals, nicotinamide is methylated by nicotinamide N-methyltransferase, producing N1-methylnicotinamide, which is subsequently excreted in urine. Research into this pathway has used the isolated chloride salt to investigate enzyme activity, methyl group transfer reactions, and regulation of nicotinamide levels in tissues. Through such studies, the compound has contributed to understanding how excess nicotinamide is processed and eliminated. In biomedical research, N1-methylnicotinamide chloride has also been employed as an analytical standard. Accurate quantification of nicotinamide metabolites in biological samples requires well-characterized reference compounds. The availability of N1-methylnicotinamide in salt form has enabled the development and validation of chromatographic and spectroscopic methods for monitoring vitamin B3 metabolism in clinical and experimental settings. These applications have supported research into nutritional status, metabolic disorders, and the pharmacokinetics of nicotinamide-related compounds. Beyond metabolism, the compound has found use in experimental studies examining the biological activity of nicotinamide derivatives. While N1-methylnicotinamide itself is generally considered biologically inactive as a vitamin, its presence and concentration can reflect changes in cellular methylation capacity and enzyme expression. As a result, it has been used as a biochemical indicator in investigations of liver function, methyl donor availability, and the effects of drugs or dietary factors on nicotinamide metabolism. In summary, N1-methylnicotinamide chloride is best understood as a chemically defined representation of a key nicotinamide metabolite. Its discovery followed from foundational work on vitamin B3 and its metabolic pathways, and its applications have centered on chemical modeling, enzymology, and analytical measurement. Although it is not used directly as a therapeutic or nutritional agent, its role in research has made it an important tool for elucidating the chemistry and biology of nicotinamide and related pyridinium compounds. References 2025. Lipid membrane composition modulates Hinokitiol's effects on keratinocytes and fibroblasts. Chemistry and Physics of Lipids. DOI: 10.1016/j.chemphyslip.2025.105511 2025. Dynamic ubiquitination networks in liver cancer: decoding E3 ligases and deubiquitinases as gatekeepers of therapeutic resistance. Medical Oncology, 42(8). DOI: 10.1007/s12032-025-02912-0 |
| Market Analysis Reports |
| List of Reports Available for N1-Methylnicotinamide chloride |