Online Database of Chemicals from Around the World
| Puyang Willing Chemical Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (21) 3422-5991 3422-5992 6480-1524 | |||
 |
wjim007@willingchem.com | |||
| Chemical manufacturer since 1988 | ||||
| chemBlink standard supplier since 2007 | ||||
| Simagchem Corporation | China | Inquire | ||
|---|---|---|---|---|
 |
+86 13806087780 | |||
 |
sale@simagchem.com | |||
| Chemical manufacturer since 2002 | ||||
| chemBlink standard supplier since 2008 | ||||
| Ningbo Actmix Polymer Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (574) 8746-8726 | |||
 |
eric.xu3000@hotmail.com | |||
| Chemical manufacturer | ||||
| chemBlink standard supplier since 2010 | ||||
| Hefei TNJ Chemical Industry Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (551) 6541-8684 | |||
 |
sales@tnjchem.com | |||
| Chemical manufacturer since 2001 | ||||
| chemBlink standard supplier since 2010 | ||||
| Jiangsu Konson Chemical Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (518) 8081-7151 | |||
 |
llchem.chris@gmail.com konson@konsonchem.cn | |||
| Chemical manufacturer since 1998 | ||||
| chemBlink standard supplier since 2020 | ||||
| Henan Rongxinxin Technology Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (392) 263-2999 | |||
 |
sales@hrongxin.xin | |||
| Chemical manufacturer since 2017 | ||||
| chemBlink standard supplier since 2023 | ||||
| Hubei Marvel-bio Medicine Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (027) 8773-8507 | |||
 |
maoerwo123@163.com | |||
 |
QQ chat | |||
 |
WeChat: kangbao813 | |||
| Chemical manufacturer since 2016 | ||||
| chemBlink standard supplier since 2025 | ||||
| Classification | Catalysts and additives >> Anti-aging agent |
|---|---|
| Name | Tetrabenzylthiuramdisulfide |
| Synonyms | Tetrabenzylthiuram disulfide; Rubber Accelerator TBzTD |
| Molecular Structure | 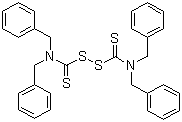 |
| Molecular Formula | C30H28N2S4 |
| Molecular Weight | 544.82 |
| CAS Registry Number | 10591-85-2 |
| EC Number | 404-310-0 |
| SMILES | C1=CC=C(C=C1)CN(CC2=CC=CC=C2)C(=S)SSC(=S)N(CC3=CC=CC=C3)CC4=CC=CC=C4 |
| Density | 1.3±0.1 g/cm3 Calc.* |
|---|---|
| Melting point | 124 ºC (Expl.) |
| Boiling point | 687.0±65.0 ºC 760 mmHg (Calc.)* |
| Flash point | 369.3±34.3 ºC (Calc.)* |
| Index of refraction | 1.713 (Calc.)* |
| * | Calculated using Advanced Chemistry Development (ACD/Labs) Software. |
| Hazard Symbols |
 GHS07 Warning Details
GHS07 Warning Details | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hazard Statements | H302-H413 Details | ||||||||||||
| Precautionary Statements | P264-P270-P273-P301+P312-P330-P501 Details | ||||||||||||
| Hazard Classification | |||||||||||||
| |||||||||||||
| SDS | Available | ||||||||||||
|
Tetrabenzylthiuram disulfide is a sulfur-containing organic compound belonging to the thiuram disulfide family. Its chemical structure consists of two thiuram moieties connected through a central disulfide linkage, with each nitrogen atom substituted with a benzyl group. The general formula can be represented as (C6H5CH2)2NCS2S2, where the central disulfide bond links two dithiocarbamate units. Compounds of this type are characterized by their ability to participate in redox reactions and to interact with metal ions and radicals, which underpins their use in various chemical and industrial applications. The discovery of thiuram disulfides traces back to research on organosulfur chemistry in the early 20th century, during which derivatives were synthesized as part of the study of vulcanization accelerators for rubber. Tetrabenzylthiuram disulfide, as a benzyl-substituted derivative, exhibits properties that differ from alkyl-substituted thiurams, including solubility in organic solvents, thermal behavior, and reactivity toward nucleophiles. The presence of the benzyl groups enhances lipophilicity, influences crystal packing, and can affect the compound's stability compared to more common dialkyl thiurams. The synthesis of tetrabenzylthiuram disulfide typically involves the reaction of benzylamines with carbon disulfide under basic conditions to form the corresponding dithiocarbamate salts. These salts are then oxidized, often using elemental sulfur or mild oxidizing agents, to generate the disulfide-linked thiuram derivative. The reaction conditions, including solvent, temperature, and choice of oxidizing agent, influence yield, purity, and the formation of side products. Careful control is required to prevent overoxidation or decomposition of the disulfide linkage. Tetrabenzylthiuram disulfide is primarily used as a reagent in organic synthesis and as a specialized chemical intermediate. Its disulfide linkage and sulfur-rich backbone allow it to act as a source of sulfur in synthetic transformations. The compound can undergo cleavage of the S–S bond under reducing conditions, generating reactive thiol or dithiocarbamate species that can participate in further chemical reactions. In addition, its aromatic substituents enable solubility in nonpolar solvents, which is beneficial for reactions requiring homogeneous conditions in organic media. In the rubber and polymer industries, thiuram disulfides, including tetrabenzylthiuram disulfide, have been explored as vulcanization accelerators and stabilizers. They enhance the crosslinking process of unsaturated polymers such as natural rubber and synthetic elastomers. The benzyl substitution pattern may alter the kinetics of vulcanization, influencing curing time, elasticity, and final mechanical properties. Compared to dialkyl thiurams, benzyl-substituted thiurams tend to exhibit higher thermal stability, which can be advantageous in certain high-temperature processing applications. From a chemical standpoint, tetrabenzylthiuram disulfide is a solid at room temperature, typically exhibiting moderate stability under ambient conditions. It is soluble in organic solvents such as chloroform, acetone, and benzene, but has limited solubility in water. Exposure to strong acids or bases can lead to hydrolysis or decomposition of the disulfide linkage, and prolonged exposure to light or heat may cause slow degradation. Handling precautions include minimizing contact with reducing agents and strong oxidizers, as these can cleave or overoxidize the disulfide bond. Tetrabenzylthiuram disulfide can also be of interest in coordination chemistry. The sulfur atoms in the dithiocarbamate moieties are capable of binding transition metals, forming complexes that may exhibit catalytic or material properties. The combination of sulfur-rich coordination sites and aromatic substituents enables the design of complexes with tailored electronic and steric properties for specialized applications in catalysis or material science. Overall, tetrabenzylthiuram disulfide is a versatile organosulfur compound that combines a central disulfide linkage with benzyl-substituted thiuram groups. Its chemical reactivity, solubility characteristics, and sulfur content make it valuable in organic synthesis, polymer processing, and coordination chemistry. The compound exemplifies how substitution patterns on thiuram disulfides can modulate physical and chemical properties, expanding their utility in both industrial and research settings. References 2025. Critical review and perspective on the production of synthetic and natural poly-�-myrcene. Polymer Bulletin. DOI: 10.1007/s00289-025-05655-0 |
| Market Analysis Reports |
| List of Reports Available for Tetrabenzylthiuramdisulfide |