Online Database of Chemicals from Around the World
| Simagchem Corporation | China | Inquire | ||
|---|---|---|---|---|
 |
+86 13806087780 | |||
 |
sale@simagchem.com | |||
| Chemical manufacturer since 2002 | ||||
| chemBlink standard supplier since 2008 | ||||
| Anhui Lixing Chemical Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (563) 816-3717 +86 13805622997 | |||
 |
hxh@lixingchem.com | |||
 |
QQ chat | |||
| Chemical manufacturer since 1992 | ||||
| chemBlink standard supplier since 2009 | ||||
| Hefei TNJ Chemical Industry Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (551) 6541-8684 | |||
 |
sales@tnjchem.com | |||
| Chemical manufacturer since 2001 | ||||
| chemBlink standard supplier since 2010 | ||||
| BOC Sciences | USA | Inquire | ||
|---|---|---|---|---|
 |
+1 (631) 485-4226 | |||
 |
info@bocsci.com | |||
| Chemical manufacturer | ||||
| chemBlink standard supplier since 2010 | ||||
| Hangzhou Leap Chem Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (571) 8771-1850 | |||
 |
market19@leapchem.com | |||
 |
QQ chat | |||
| Chemical manufacturer since 2006 | ||||
| chemBlink standard supplier since 2015 | ||||
| Neostar United (Changzhou) Industrial Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (519) 8555-7386 +86 18015025600 | |||
 |
marketing1@neostarunited.com | |||
| Chemical distributor since 2014 | ||||
| chemBlink standard supplier since 2020 | ||||
| Auben Chemical Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 19121901603 | |||
 |
sales@aubenchem.com | |||
| Chemical distributor since 2023 | ||||
| chemBlink standard supplier since 2025 | ||||
| Santa Cruz Biotechnology, Inc. | USA | Inquire | ||
|---|---|---|---|---|
 |
+1 (831) 457-3800 | |||
 |
scbt@scbt.com | |||
| Chemical manufacturer | ||||
| Classification | Organic raw materials >> Ether compounds and their derivatives >> Ether, ether alcohol |
|---|---|
| Name | 2-Methoxyethyl ether |
| Synonyms | Bis(2-methoxyethyl) ether; Diethylene glycol dimethyl ether |
| Molecular Structure | 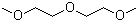 |
| Molecular Formula | C6H14O3 |
| Molecular Weight | 134.17 |
| CAS Registry Number | 111-96-6 |
| EC Number | 203-924-4 |
| SMILES | COCCOCCOC |
| Density | 0.9±0.1 g/cm3 Calc.*, 0.944 g/mL (Expl.) |
|---|---|
| Melting point | -64 ºC (Expl.) |
| Boiling point | 159.8 ºC 760 mmHg (Calc.)*, 161 - 162 ºC (Expl.) |
| Flash point | 70.0 ºC (Calc.)*, 57 ºC (Expl.) |
| Solubility | water: Miscible (Expl.) |
| Index of refraction | 1.394 (Calc.)*, 1.408 (Expl.) |
| * | Calculated using Advanced Chemistry Development (ACD/Labs) Software. |
| Hazard Symbols |

 GHS02;GHS08 DangerGHS02 Details
GHS02;GHS08 DangerGHS02 Details | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hazard Statements | H226-H360FD Details | ||||||||||||||||||||||||||||
| Precautionary Statements | P203-P210-P233-P240-P241-P242-P243-P280-P303+P361+P353-P318-P370+P378-P403+P235-P405-P501 Details | ||||||||||||||||||||||||||||
| Hazard Classification | |||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||
| Transport Information | UN 1993;UN 3271 | ||||||||||||||||||||||||||||
| SDS | Available | ||||||||||||||||||||||||||||
|
2‑Methoxyethyl ether is a small organic ether characterized by the presence of an ether linkage connecting a two-carbon chain to a methoxy group. Its chemical formula is C3H8O2, and it can be structurally described as CH3OCH2CH2OH in its monomeric form, depending on the context of naming, although the term 2‑methoxyethyl ether often refers to its use as a functionalized ether solvent or intermediate. The ether functional group confers chemical stability and low reactivity under neutral conditions, while the adjacent methylene groups influence solubility, volatility, and miscibility with organic solvents and water. The discovery of 2‑methoxyethyl ether stems from the broader study of glycol ethers and low molecular weight ethers in the early to mid-20th century, as chemists sought organic solvents with balanced polarity, chemical inertness, and low volatility. These ethers were found to be effective as reaction media for a wide range of organic transformations, particularly where moderate polarity was required to dissolve both hydrophilic and lipophilic substrates. The methoxy group imparts slight polarity, while the ether oxygen reduces flammability and provides hydrogen bonding interactions in specific chemical contexts. 2‑Methoxyethyl ether is commonly synthesized by the alkylation of 2‑methoxyethanol or its derivatives with suitable halides under basic conditions. For example, the reaction of 2‑methoxyethanol with an alkyl halide in the presence of a base such as sodium hydride or potassium carbonate leads to the formation of the ether via nucleophilic substitution. Alternative methods involve Williamson ether synthesis, which employs the deprotonated alcohol as a nucleophile attacking an electrophilic alkyl halide. Control over reaction conditions such as temperature, stoichiometry, and solvent is critical to maximize yield and minimize side reactions such as elimination or polymerization. Chemically, 2‑methoxyethyl ether exhibits the typical reactivity of an ether functional group. It is relatively unreactive toward acids, bases, and oxidizing agents under mild conditions. However, under strong acidic conditions, ethers can undergo cleavage, yielding the corresponding alcohols and alkyl halides. The ether linkage also provides stability against hydrolysis, making the compound suitable for use as a solvent in reactions that involve water-sensitive reagents. Its methoxyethyl fragment contributes to moderate polarity and good solvation of polar compounds, including salts and hydrogen-bonding molecules. In organic synthesis, 2‑methoxyethyl ether is often employed as a solvent, intermediate, or reagent in the preparation of more complex molecules. Its polarity and miscibility profile enable it to act as a medium for nucleophilic substitution, acylation, and condensation reactions. It has also been used in the preparation of glycosidic derivatives, polymer precursors, and as a protecting group in multistep synthetic sequences where the ether oxygen can later be removed or functionalized. Its low reactivity and solvent properties make it particularly valuable in processes that require mild, inert media. From a physical perspective, 2‑methoxyethyl ether is a colorless, low-viscosity liquid with moderate volatility. It is miscible with a range of organic solvents such as alcohols, ethers, and ketones, and exhibits limited solubility in water depending on temperature. The compound has a relatively low boiling point, allowing for distillation and purification under standard laboratory conditions. Care should be taken when handling it because ethers can form peroxides over time, especially upon prolonged exposure to air and light. Proper storage in airtight containers and stabilization with inhibitors is recommended to prevent peroxide formation. In industrial and laboratory applications, 2‑methoxyethyl ether serves as a versatile solvent and reagent due to its combination of chemical stability, moderate polarity, and ability to dissolve a broad spectrum of organic and inorganic compounds. It enables selective reactions without participating as a nucleophile under standard conditions and is compatible with many metal-catalyzed or acid-sensitive transformations. Its dual character as both polar and aprotic provides flexibility for chemists in designing synthetic strategies and solvent systems. Overall, 2‑methoxyethyl ether is a chemically stable, moderately polar ether that functions as a solvent and intermediate in organic synthesis. Its low reactivity, miscibility properties, and ability to solubilize diverse compounds make it a valuable tool in laboratories and industrial processes, while its methoxyethyl group contributes to both solubility and chemical versatility. References 2025. Solvent co-intercalation in layered cathode active materials for sodium-ion batteries. Nature Materials. DOI: 10.1038/s41563-025-02287-7 2025. Efficient and stable synthesis of diethylene glycol dimethyl ether over aluminophosphate catalyst. Research on Chemical Intermediates. DOI: 10.1007/s11164-025-05667-5 |
| Market Analysis Reports |
| List of Reports Available for 2-Methoxyethyl ether |