Online Database of Chemicals from Around the World
| RC Chemicals Lab Inc | USA | Inquire | ||
|---|---|---|---|---|
 |
+1 (401) 232-4508 | |||
 |
info@rcchemicallabs.net | |||
| Chemical distributor since 2010 | ||||
| chemBlink standard supplier since 2025 | ||||
| Classification | Analytical chemistry >> Standard >> Standard material |
|---|---|
| Name | 1-Pentyl-3-(1-naphthoyl)indole |
| Molecular Structure | 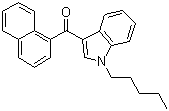 |
| Molecular Formula | C24H23NO |
| Molecular Weight | 341.44 |
| CAS Registry Number | 209414-07-3 |
| EC Number | 635-711-0 |
| SMILES | CCCCCN1C=C(C2=CC=CC=C21)C(=O)C3=CC=CC4=CC=CC=C43 |
| Density | 1.1±0.1 g/cm3 Calc.* |
|---|---|
| Boiling point | 534.2±23.0 ºC 760 mmHg (Calc.)* |
| Flash point | 276.9±22.6 ºC (Calc.)* |
| Solubility | Soluble DMSO: 100 mM, ethanol :100 mM (Expl.) |
| Index of refraction | 1.606 (Calc.)* |
| * | Calculated using Advanced Chemistry Development (ACD/Labs) Software. |
| Hazard Symbols |


 GHS02;GHS06;GHS08 Danger Details
GHS02;GHS06;GHS08 Danger Details | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hazard Statements | H225-H301+H311+H331-H370 Details | ||||||||||||||||
| Precautionary Statements | P210-P280-P301+P310+P330-P302+P352+P312-P304+P340+P311 Details | ||||||||||||||||
| Hazard Classification | |||||||||||||||||
| |||||||||||||||||
| SDS | Available | ||||||||||||||||
|
1‑Pentyl‑3-(1‑naphthoyl)indole is a synthetic indole derivative characterized by a pentyl substituent at the nitrogen atom of the indole ring and a 1-naphthoyl group at the third position of the indole core. Its molecular formula is C23H23NO, and it is a lipophilic compound with limited solubility in water but good solubility in organic solvents such as ethanol, methanol, and dimethyl sulfoxide. The combination of the indole core with the naphthoyl substituent imparts significant aromaticity, hydrophobic character, and the potential for strong π-π stacking interactions, which influence its physical and chemical behavior. The discovery of 1‑pentyl‑3-(1‑naphthoyl)indole is linked to the exploration of synthetic cannabinoids and related indole derivatives. Compounds of this type were developed to investigate the structure-activity relationships of cannabinoids and their interaction with cannabinoid receptors. The indole nucleus provides a versatile scaffold that allows functionalization at the nitrogen and third positions, while the naphthoyl group contributes hydrophobicity and electronic effects that influence receptor binding. These structural features have made 1‑pentyl‑3-(1‑naphthoyl)indole a subject of research in pharmacology and medicinal chemistry. Synthesis of 1‑pentyl‑3-(1‑naphthoyl)indole typically begins with indole as the starting material. N‑alkylation with a pentyl halide under basic conditions introduces the pentyl substituent at the nitrogen atom. Subsequent acylation at the third position of the indole ring with 1‑naphthoyl chloride produces the target compound. Reaction conditions are carefully controlled to achieve selective N-alkylation and 3-acylation without overreaction or side products. Purification is commonly performed by recrystallization or chromatography to isolate the pure product for chemical or biological studies. Chemically, 1‑pentyl‑3-(1‑naphthoyl)indole exhibits typical reactivity of indole derivatives. The carbonyl group of the naphthoyl moiety can undergo nucleophilic addition, reduction, or condensation reactions. The indole ring itself is reactive toward electrophilic substitution at the C2 and C5 positions, allowing further functionalization. The pentyl group provides steric hindrance and lipophilicity, influencing both the solubility and the selectivity of chemical reactions. These properties enable the compound to be used as a building block in synthetic studies and as a reference compound in receptor-binding research. From a pharmacological perspective, 1‑pentyl‑3-(1‑naphthoyl)indole acts as a cannabinoid receptor agonist, interacting primarily with CB1 and CB2 receptors. The presence of the pentyl chain and naphthoyl group enhances receptor affinity and lipophilicity, allowing the compound to cross lipid membranes efficiently. This pharmacological profile has made it a valuable tool for studying receptor mechanisms, structure-activity relationships, and the effects of synthetic cannabinoids on biological systems. It has also contributed to research on therapeutic applications and toxicology studies of cannabinoid analogues. In terms of physical properties, the compound is a crystalline solid or viscous oil depending on purity and temperature. It is sensitive to prolonged light exposure and strong oxidizing agents, which may cause degradation of the indole or carbonyl functionalities. Handling precautions include using gloves, goggles, and working in well-ventilated environments to prevent inhalation or skin contact. Its chemical stability under inert conditions facilitates storage and handling in laboratory environments. Overall, 1‑pentyl‑3-(1‑naphthoyl)indole is a highly functionalized indole derivative combining a lipophilic pentyl group with an aromatic naphthoyl substituent. Its chemical reactivity, lipophilicity, and receptor-binding properties make it a versatile compound for synthetic, pharmacological, and receptor-based studies. The molecule serves as a key scaffold for the development of cannabinoid analogues and the exploration of structure-activity relationships, supporting ongoing research in medicinal chemistry, pharmacology, and chemical biology. References 2025. Repeated treatment with JWH-018 progressively increases motor activity and aggressiveness in male mice: involvement of CB1 cannabinoid and D1/D2 dopaminergic receptors. European Journal of Pharmacology. DOI: 10.1016/j.ejphar.2025.177633 2025. Synthetic cannabinoid receptor agonists exacerbate fentanyl-elicited respiratory depression and confer resistance to naloxone rescue in mice. Drug and Alcohol Dependence. DOI: 10.1016/j.drugalcdep.2025.112672 2025. Pharmaco-toxicological effects of the synthetic cannabinoids 4F-ABUTINACA, SDB-005, and JWH-018 in mice. In vitro and in vivo studies. European Journal of Pharmacology. DOI: 10.1016/j.ejphar.2025.177586 |
| Market Analysis Reports |
| List of Reports Available for 1-Pentyl-3-(1-naphthoyl)indole |