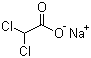
Sodium dichloroacetate is the sodium salt of dichloroacetic acid, a small halogenated carboxylate that has been studied extensively for its biochemical and pharmacological properties. The compound consists of a dichloromethyl group attached to a carboxylate anion, balanced by a sodium cation. Its scientific importance lies primarily in its role as a metabolic modulator and as a tool compound in studies of mitochondrial function and intermediary metabolism.
The discovery of dichloroacetic acid dates back to nineteenth-century investigations into chlorinated acetic acids, which were prepared during systematic explorations of halogen substitution reactions in simple organic acids. As methods for chlorination and purification improved, dichloroacetic acid and its salts, including sodium dichloroacetate, became well-defined chemical entities. Early interest centered on their chemical properties, acidity, and reactivity, which were compared with those of mono- and trichloroacetic acids to understand the effects of halogen substitution on carboxylic acids.
Sodium dichloroacetate later gained attention in biological and medical research due to its influence on cellular metabolism. In the mid-twentieth century, researchers studying carbohydrate metabolism identified dichloroacetate as an inhibitor of pyruvate dehydrogenase kinase. This inhibition leads to activation of the pyruvate dehydrogenase complex, promoting the conversion of pyruvate to acetyl-CoA and enhancing mitochondrial oxidation of glucose-derived carbon. The sodium salt form was favored in experimental work because of its high water solubility and ease of administration in aqueous systems.
The application of sodium dichloroacetate as a research tool expanded as interest grew in metabolic regulation and mitochondrial dysfunction. It has been widely used in laboratory studies to probe the balance between glycolysis and oxidative phosphorylation. By shifting cellular metabolism toward increased mitochondrial activity, sodium dichloroacetate has helped elucidate mechanisms underlying metabolic diseases, ischemia, and altered energy metabolism in various cell types. Its effects have been studied in isolated mitochondria, cultured cells, and animal models to better understand metabolic control points.
In addition to its role in metabolism research, sodium dichloroacetate has been investigated for potential therapeutic applications. Its ability to modify metabolic pathways prompted studies in conditions associated with impaired mitochondrial function or abnormal glucose metabolism. These investigations have primarily been conducted in experimental and clinical research settings, where the compound served as a pharmacological probe to test hypotheses about disease mechanisms rather than as an established treatment. Through this work, sodium dichloroacetate contributed to a broader appreciation of metabolism as a target for therapeutic intervention.
From a chemical and industrial perspective, sodium dichloroacetate has also been used as an intermediate and reagent. Dichloroacetate salts have found applications in organic synthesis, particularly in reactions where controlled acidity or the presence of a halogenated carboxylate is advantageous. In environmental and analytical chemistry, dichloroacetate has been studied as a byproduct of water chlorination processes, making sodium dichloroacetate relevant to investigations of water treatment and disinfection byproducts.
Safety and toxicological studies have accompanied the increased use of sodium dichloroacetate in research. These studies have examined its metabolism, distribution, and potential adverse effects, providing important context for its handling and experimental use. Such evaluations have reinforced the need for controlled dosing and careful interpretation of results when the compound is used in biological systems.
In summary, sodium dichloroacetate is a chemically simple yet biologically influential compound whose significance arises from its ability to modulate key metabolic pathways. From its origins in classical organic chemistry to its applications in modern metabolic research, it has served as an important tool for understanding mitochondrial function and energy metabolism. Its continued use reflects the enduring value of small, well-characterized molecules in advancing biochemical and physiological knowledge.
References
2025. Myocardial pyruvate dehydrogenase kinase 4 drives sex-specific cardiac responses to endotoxemia. JCI Insight.
DOI: 10.1172/jci.insight.191649
2025. 1,8-naphthalimide-based DNA intercalators and anticancer agents: a systematic review. Molecular Diversity.
DOI: 10.1007/s11030-025-11251-1
|


















 GHS07;GHS08 Warning Details
GHS07;GHS08 Warning Details