Online Database of Chemicals from Around the World
| Shanghai Worldyang Chemical Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 13651600618 +86 (21) 5679-5779 | |||
 |
sales7777@worldyachem.com | |||
 |
QQ chat | |||
 |
WeChat: 13651600618 | |||
 |
WhatsApp: +86 13651600618 | |||
| Chemical manufacturer since 2012 | ||||
| chemBlink premium supplier since 2023 | ||||
| Hangzhou Verychem Science And Technology Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (571) 8816-2785 +86 13606544505 | |||
 |
lucy@verychem.com | |||
| Chemical manufacturer since 2004 | ||||
| chemBlink massive supplier since 2021 | ||||
| Classification | Biochemical >> Nucleoside drugs >> Nucleotides and their analogues |
|---|---|
| Name | 1,2,4-Triazole |
| Synonyms | 1,2,4-1H-Triazole |
| Molecular Structure | 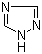 |
| Molecular Formula | C2H3N3 |
| Molecular Weight | 69.07 |
| CAS Registry Number | 288-88-0 |
| EC Number | 206-022-9 |
| SMILES | C1=NC=NN1 |
| Density | 1.3±0.1 g/cm3 Calc.* |
|---|---|
| Melting point | 119 - 121 ºC (Expl.) |
| Boiling point | 260.0±9.0 ºC 760 mmHg (Calc.)*, 260 ºC (Expl.) |
| Flash point | 139.1±11.7 ºC (Calc.)*, 170 ºC (Expl.) |
| Solubility | water: 1250 g/L (20 ºC) (Expl.) |
| Index of refraction | 1.535 (Calc.)* |
| * | Calculated using Advanced Chemistry Development (ACD/Labs) Software. |
| Hazard Symbols |

 GHS07;GHS08 Danger Details
GHS07;GHS08 Danger Details | ||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hazard Statements | H302-H319-H360FD Details | ||||||||||||||||||||||||||||||||||||||||||||||||
| Precautionary Statements | P203-P264-P264+P265-P270-P280-P301+P317-P305+P351+P338-P318-P330-P337+P317-P405-P501 Details | ||||||||||||||||||||||||||||||||||||||||||||||||
| Hazard Classification | |||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||
| SDS | Available | ||||||||||||||||||||||||||||||||||||||||||||||||
|
1,2,4-Triazole is a five-membered heterocyclic compound containing three nitrogen atoms at positions 1, 2, and 4 of the ring, with the molecular formula C2H3N3. It is a colorless to white crystalline solid under standard conditions and is highly soluble in water and polar organic solvents due to the presence of multiple nitrogen atoms capable of hydrogen bonding. The compound exhibits aromaticity, which imparts chemical stability and contributes to its reactivity in nucleophilic and electrophilic substitution reactions. The discovery of 1,2,4-triazole dates back to early studies in heterocyclic chemistry, where researchers explored nitrogen-rich ring systems for their potential in pharmaceuticals, agriculture, and materials science. Its structure provides a versatile scaffold that can be functionalized at various positions on the ring, enabling the synthesis of derivatives with diverse biological and chemical properties. The electron-rich nature of the ring also makes it suitable for coordination with metal ions, which has led to applications in catalysis and materials chemistry. Synthesis of 1,2,4-triazole can be achieved through multiple methods. A common approach involves the cyclization of hydrazine derivatives with formamide or formic acid under heating conditions. Alternative synthetic routes include reactions of thiosemicarbazides with nitriles or other carbonyl compounds to form the triazole ring. Reaction conditions, such as temperature, solvent, and pH, are carefully optimized to maximize yield and minimize side products. The resulting 1,2,4-triazole is typically purified by recrystallization or distillation. Chemically, 1,2,4-triazole exhibits properties typical of aromatic nitrogen heterocycles. It can act as a nucleophile, coordinating with electrophilic centers, or as a ligand in metal complex formation. The nitrogen atoms in the ring are involved in tautomeric equilibria, which can influence reactivity and hydrogen bonding. The compound is also stable under acidic and basic conditions, making it suitable for a variety of chemical transformations. 1,2,4-Triazole is widely used as a building block in pharmaceuticals, agrochemicals, and coordination chemistry. In medicinal chemistry, triazole derivatives serve as antifungal, antibacterial, antiviral, and anticancer agents, owing to their ability to interact with biological targets such as enzymes and nucleic acids. In agriculture, triazole derivatives are employed as fungicides and plant growth regulators. Additionally, 1,2,4-triazole can be incorporated into polymers and metal-organic frameworks, enhancing material properties such as thermal stability, coordination capacity, and electronic behavior. From a physical perspective, 1,2,4-triazole has a melting point around 120–122°C and is thermally stable under normal laboratory conditions. Its solubility in water and polar solvents facilitates its handling and incorporation into chemical reactions. The compound is relatively low in toxicity but should be handled with standard safety precautions, including gloves and eye protection, to avoid inhalation or skin contact. Overall, 1,2,4-triazole is a versatile nitrogen-containing heterocyclic compound with significant chemical and biological importance. Its aromatic structure, functionalizable nitrogen atoms, and stability under various conditions make it a valuable scaffold for synthetic chemistry. The compound supports applications in pharmaceuticals, agriculture, and materials science, serving as a key intermediate in the development of bioactive molecules, coordination complexes, and advanced materials. References 2025. Process for preparing azoles. CN Patent, 120554331. 2025. Nanocomposite catalyst for water splitting. US Patent, 12416085. 2025. Preparation method of posaconazole starting material SM3. CN Patent, 120040313. |
| Market Analysis Reports |
| List of Reports Available for 1,2,4-Triazole |