Online Database of Chemicals from Around the World
| Hangzhou Cyanochem Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (571) 8522-0831 +86 17788583750 | |||
 |
info@cyanochem.com | |||
 |
Skype Chat | |||
 |
QQ chat | |||
| Chemical manufacturer since 2017 | ||||
| chemBlink standard supplier since 2018 | ||||
| Henan Pharma Technology Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (371) 6336-4608 | |||
 |
sales1@cnpharma-tech.com | |||
| Chemical distributor since 2018 | ||||
| chemBlink standard supplier since 2018 | ||||
| RC Chemicals Lab Inc | USA | Inquire | ||
|---|---|---|---|---|
 |
+1 (401) 232-4508 | |||
 |
info@rcchemicallabs.net | |||
| Chemical distributor since 2010 | ||||
| chemBlink standard supplier since 2025 | ||||
| Cayman Chemical Company | USA | Inquire | ||
|---|---|---|---|---|
 |
+1 (734) 971-3335 | |||
 |
sales@caymanchem.com | |||
| Chemical manufacturer | ||||
| Classification | API >> Nervous system medication >> Other nervous system medication |
|---|---|
| Name | 7-Chloro-5-(2-chlorophenyl)-1,3-dihydro-1-methyl-2H-1,4-benzodiazepin-2-one |
| Synonyms | Chlorodiazepam; Ro 5-3448 |
| Molecular Structure | 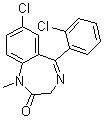 |
| Molecular Formula | C16H12Cl2N2O |
| Molecular Weight | 319.18 |
| CAS Registry Number | 2894-68-0 |
| SMILES | CN1C(=O)CN=C(C2=C1C=CC(=C2)Cl)C3=CC=CC=C3Cl |
| Density | 1.4±0.1 g/cm3 Calc.* |
|---|---|
| Melting point | 217-219 ººC (dichloromethane ethyl ether ) (Expl.)** |
| Boiling point | 524.6±50.0 ºC 760 mmHg (Calc.)* |
| Flash point | 271.1±30.1 ºC (Calc.)* |
| Solubility | water: 10 mg/mL, DMSO 2 mg/mL (Expl.) |
| Index of refraction | 1.647 (Calc.)* |
| * | Calculated using Advanced Chemistry Development (ACD/Labs) Software V11.02 (©1994-2013 ACD/Labs) |
| ** | Reeder, Earl; BE 629227 1963. |
| Hazard Symbols |

 GHS06;GHS09 Danger Details
GHS06;GHS09 Danger Details |
|---|---|
| Hazard Statements | H301+H311-H301-H311-H410 Details |
| Precautionary Statements | P262-P264-P270-P273-P280-P301+P316-P302+P352-P316-P321-P330-P361+P364-P391-P405-P501 Details |
| SDS | Available |
|
7-Chloro-5-(2-chlorophenyl)-1,3-dihydro-1-methyl-2H-1,4-benzodiazepin-2-one is a member of the benzodiazepine class of compounds, characterized by a fused benzene and seven-membered diazepine ring system. Its molecular formula is C16H12Cl2N2O, and it typically appears as a crystalline solid with low solubility in water but good solubility in polar organic solvents such as ethanol, methanol, and dimethyl sulfoxide. The compound contains two chlorine substituents—one on the benzodiazepine core at position 7 and one on the phenyl group at position 5—which influence both its electronic distribution and steric properties. The discovery of this compound is part of the broader development of benzodiazepine derivatives in medicinal chemistry. Benzodiazepines were first identified for their anxiolytic, sedative, muscle relaxant, and anticonvulsant activities. Substitution at the 5-position with aryl groups and modification of the N1-methyl group have been extensively explored to tune pharmacokinetic and pharmacodynamic properties. The chlorine substituents increase lipophilicity, enhance receptor binding affinity, and contribute to metabolic stability. Synthesis of 7-chloro-5-(2-chlorophenyl)-1,3-dihydro-1-methyl-2H-1,4-benzodiazepin-2-one typically involves the condensation of 2-amino-5-chlorobenzophenone derivatives with appropriate amides or methylated precursors under cyclization conditions. The key steps include formation of the diazepine ring via intramolecular condensation and selective introduction of the chlorine substituents at defined positions on the aromatic rings. Reaction parameters such as temperature, solvent, and pH are carefully controlled to maximize yield and avoid side reactions, and the final product is purified by recrystallization or chromatography. Chemically, this benzodiazepine derivative exhibits typical reactivity of amides and aromatic systems. The carbonyl group at the 2-position can participate in hydrogen bonding and nucleophilic interactions, while the diazepine nitrogen atoms contribute to tautomerism and receptor binding. The aromatic chlorides influence electronic properties and can be used as sites for further functionalization, such as halogen exchange or cross-coupling reactions for structural diversification. The N1-methyl group enhances lipophilicity and influences the compound’s metabolic profile. Pharmacologically, 7-chloro-5-(2-chlorophenyl)-1,3-dihydro-1-methyl-2H-1,4-benzodiazepin-2-one acts on the central nervous system by modulating the activity of gamma-aminobutyric acid type A (GABAA) receptors. Binding to the benzodiazepine site of the receptor enhances the effect of GABA, resulting in increased chloride ion influx, hyperpolarization of neurons, and subsequent anxiolytic, sedative, and anticonvulsant effects. Structural modifications, such as chlorine substitution and N1-methylation, affect potency, duration of action, and receptor selectivity. Physically, the compound is thermally stable under standard laboratory conditions and typically requires protection from strong acids or bases that may hydrolyze the amide functionality. Its crystalline form facilitates storage and handling, and solubility in polar organic solvents allows its use in formulation and chemical derivatization. Safety considerations include avoiding inhalation of dust or direct contact with skin and eyes, as with many synthetic benzodiazepines. Overall, 7-chloro-5-(2-chlorophenyl)-1,3-dihydro-1-methyl-2H-1,4-benzodiazepin-2-one is a chemically and pharmacologically significant benzodiazepine derivative. Its rigid fused ring system, chlorine substitutions, and methylated nitrogen contribute to its receptor-binding properties, metabolic stability, and lipophilicity. The compound serves as a key scaffold in medicinal chemistry for the development of central nervous system-active agents, supporting applications in anxiolytic, sedative, and anticonvulsant therapies. References 2024. Discriminative stimulus and reinforcing effects of diclazepam in rodents. Pharmacology, Biochemistry, and Behavior, 231. DOI: 10.1016/j.pbb.2023.173687 |
| Market Analysis Reports |
| List of Reports Available for 7-Chloro-5-(2-chlorophenyl)-1,3-dihydro-1-methyl-2H-1,4-benzodiazepin-2-one |