Online Database of Chemicals from Around the World
| Novasyn Organics Pvt. Ltd. | India | Inquire | ||
|---|---|---|---|---|
 |
+91 (406) 620-2233 633-9111 | |||
 |
info@novasynorganics.com | |||
| Chemical manufacturer | ||||
| chemBlink standard supplier since 2010 | ||||
| Neostar United (Changzhou) Industrial Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (519) 8555-7386 +86 18015025600 | |||
 |
marketing1@neostarunited.com | |||
| Chemical distributor since 2014 | ||||
| chemBlink standard supplier since 2020 | ||||
| Auben Chemical Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 19121901603 | |||
 |
sales@aubenchem.com | |||
| Chemical distributor since 2023 | ||||
| chemBlink standard supplier since 2025 | ||||
| Classification | Pharmaceutical intermediate >> Heterocyclic compound intermediate >> Pyridine compound >> Methylpyridine |
|---|---|
| Name | 5-Chloro-2-fluoro-3-methylpyridine |
| Synonyms | 5-Chloro-2-fluoro-3-picoline; 2-Fluoro-3-methyl-5-chloropyridine |
| Molecular Structure | 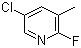 |
| Molecular Formula | C6H5ClFN |
| Molecular Weight | 145.56 |
| CAS Registry Number | 375368-84-6 |
| EC Number | 675-616-1 |
| SMILES | CC1=CC(=CN=C1F)Cl |
| Density | 1.3±0.1 g/cm3 Calc.*, 1.204 - 1.324 g/mL (Expl.) |
|---|---|
| Boiling point | 189.4±35.0 ºC 760 mmHg (Calc.)* |
| Flash point | 68.3±25.9 ºC (Calc.)* |
| Index of refraction | 1.504 (Calc.)* |
| * | Calculated using Advanced Chemistry Development (ACD/Labs) Software. |
| Hazard Symbols |

 GHS05;GHS07 Danger Details
GHS05;GHS07 Danger Details | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hazard Statements | H302-H315-H318-H319-H335 Details | ||||||||||||||||||||||||||||
| Precautionary Statements | P261-P264-P264+P265-P270-P271-P280-P301+P317-P302+P352-P304+P340-P305+P351+P338-P305+P354+P338-P317-P319-P321-P330-P332+P317-P337+P317-P362+P364-P403+P233-P405-P501 Details | ||||||||||||||||||||||||||||
| Hazard Classification | |||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||
| SDS | Available | ||||||||||||||||||||||||||||
|
5-Chloro-2-fluoro-3-methylpyridine is a heteroaromatic compound belonging to the substituted pyridine family, with chlorine, fluorine, and methyl groups positioned at the 5, 2, and 3 locations of the pyridine ring, respectively. Its molecular formula is C6H5ClFN. The compound typically appears as a colorless to pale yellow liquid and exhibits solubility in organic solvents such as ethanol, dichloromethane, and acetone, while showing limited solubility in water due to its hydrophobic substituents and aromatic ring. The discovery and development of 5-chloro-2-fluoro-3-methylpyridine are linked to the broader exploration of substituted pyridines in medicinal chemistry and agrochemical research. Substituted pyridines are versatile building blocks that can be functionalized to create pharmacologically active compounds, ligands for catalysis, and intermediates in organic synthesis. The presence of both electron-withdrawing halogens and an electron-donating methyl group allows fine-tuning of the electronic properties of the pyridine ring, which is critical for subsequent chemical transformations. Synthesis of 5-chloro-2-fluoro-3-methylpyridine is generally achieved through selective halogenation of 3-methylpyridine derivatives. One approach involves starting from 3-methylpyridine, followed by regioselective chlorination at the 5-position and fluorination at the 2-position using appropriate halogenating reagents under controlled temperature and solvent conditions. Reaction conditions are optimized to prevent over-halogenation or substitution at undesired positions. Purification is typically carried out using distillation or chromatography to obtain the target compound in high purity. Chemically, 5-chloro-2-fluoro-3-methylpyridine exhibits the typical reactivity of halogenated pyridines. The halogen atoms act as electron-withdrawing groups, making the ring less nucleophilic and more prone to nucleophilic aromatic substitution, especially at positions activated by the substituents. The methyl group at the 3-position exerts a modest electron-donating effect through hyperconjugation, slightly modulating the electronic distribution of the ring. The compound can undergo cross-coupling reactions, substitution reactions, or lithiation followed by functionalization, making it a versatile intermediate in organic synthesis. In practical applications, 5-chloro-2-fluoro-3-methylpyridine is used primarily as an intermediate in the synthesis of pharmaceuticals, agrochemicals, and fine chemicals. Its substituted pyridine core serves as a scaffold for constructing more complex molecules, where the halogen atoms can be further modified to introduce functional groups such as amines, ethers, or additional heterocycles. The electronic and steric properties imparted by the halogens and methyl group allow chemists to design compounds with specific binding or reactivity profiles. From a physical perspective, the compound is stable under normal laboratory conditions but should be stored in a cool, dry environment to prevent hydrolysis or decomposition. Standard safety measures should be taken, including the use of gloves, goggles, and adequate ventilation, as halogenated pyridines can be irritants and may have toxic effects upon prolonged exposure. Overall, 5-chloro-2-fluoro-3-methylpyridine is a chemically and industrially significant substituted pyridine. Its combination of halogen substituents and a methyl group provides electronic tuning and reactivity that make it a versatile intermediate in organic synthesis. The compound is widely utilized in the preparation of pharmaceuticals, agrochemicals, and other heterocyclic derivatives, highlighting its importance as a building block in chemical research and industrial applications. References 2021. Triazole carbamate pyridyl sulfonamides as lpa receptor antagonists and uses thereof. WO Patent. URL: WO-2021097039-A1 2020. Erk inhibitor and use thereof. WO Patent. URL: WO-2019233457-A1 |
| Market Analysis Reports |
| List of Reports Available for 5-Chloro-2-fluoro-3-methylpyridine |