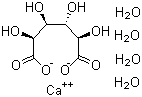
Calcium D-saccharate tetrahydrate is an inorganic–organic salt formed from calcium ions and D-saccharic acid, crystallized with four molecules of water. D-Saccharic acid is a dicarboxylic acid produced by the oxidation of glucose, and its calcium salt has been studied primarily in relation to carbohydrate chemistry, mineral metabolism, and nutritional science. The compound is best understood as a stable, isolable form of a naturally derived sugar acid coordinated to calcium.
The historical background of calcium D-saccharate tetrahydrate is closely tied to early investigations of carbohydrate oxidation products. In the nineteenth century, chemists exploring the chemistry of sugars observed that strong oxidation of glucose yielded saccharic acids with distinct chemical behavior compared with the parent monosaccharide. As analytical and preparative methods improved, D-saccharic acid was isolated and characterized, and its ability to form salts with alkaline earth metals became apparent. Calcium salts were of particular interest because of their relatively low solubility and well-defined crystalline nature, which facilitated purification and study.
The preparation of calcium D-saccharate tetrahydrate typically involves neutralization of D-saccharic acid with a calcium source under aqueous conditions, followed by controlled crystallization. The incorporation of water molecules into the crystal lattice reflects the strong hydrogen-bonding capacity of the saccharate anion and is characteristic of many carbohydrate-derived salts. Early structural studies focused on understanding how the multiple hydroxyl and carboxylate groups of D-saccharate coordinate calcium ions, providing insight into metal–ligand interactions in polyfunctional organic acids.
Scientific interest in calcium D-saccharate expanded during the twentieth century as the metabolism of carbohydrates and their oxidation products became better understood. D-Saccharic acid and its salts were recognized as metabolites that could arise from the breakdown of glucuronic acid derivatives. This connection linked calcium D-saccharate tetrahydrate to broader biochemical pathways involving detoxification and conjugation reactions in living organisms. As a result, the compound became relevant in studies examining how carbohydrate-derived acids interact with minerals in biological systems.
One of the most notable applications of calcium D-saccharate has been in nutritional and biochemical research. The compound has been investigated for its influence on enzymatic processes related to glucuronidation, a major pathway for the metabolism and elimination of endogenous compounds and xenobiotics. In experimental settings, calcium D-saccharate has been used as a source of D-saccharate to study how modulation of these pathways affects the balance of conjugated and free metabolites. The calcium salt form offers advantages in handling and stability compared with the free acid.
In addition to its role in metabolic research, calcium D-saccharate tetrahydrate has been of interest in coordination chemistry and materials science. The saccharate ligand, with its multiple oxygen donors, provides a versatile framework for binding metal ions. Studies of calcium D-saccharate contributed to understanding how sugar acids can act as chelating agents and how hydration influences crystal structure and stability. These investigations have informed broader research on metal complexes of polyhydroxy carboxylates.
The compound has also been used as a reference material in analytical chemistry. Its defined composition and predictable hydration state make it suitable for calibrating methods used to quantify saccharic acid derivatives or calcium content in complex matrices. Such applications underscore its utility as a chemically well-characterized standard rather than as a bulk industrial material.
Overall, calcium D-saccharate tetrahydrate occupies a specialized but meaningful position in chemical and biochemical research. Its discovery followed from foundational work on carbohydrate oxidation, and its applications have spanned metabolism studies, coordination chemistry, and analytical science. While it is not widely used in large-scale industrial processes, its value lies in providing a stable and informative model for exploring the interactions between sugar-derived acids and metal ions, as well as their roles in biological systems.
References
2025. A metabolic-engineering framework approach via fed-batch fermentation for enhancing glucaric acid production in Komagataella phaffii. Enzyme and Microbial Technology.
DOI: 10.1016/j.enzmictec.2025.110627
|
















 GHS07 Warning Details
GHS07 Warning Details