Online Database of Chemicals from Around the World
| Capot Chemical Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (571) 8558-6718 +86 13336195806 | |||
 |
capotchem@gmail.com sales@capotchem.com | |||
 |
QQ chat | |||
| Chemical manufacturer | ||||
| chemBlink standard supplier since 2006 | ||||
| Shanghai CR Corporation Limited | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (21) 5736-6208 +86 13917420128 | |||
 |
fred.wen@crcorporation.cn | |||
 |
QQ chat | |||
| Chemical manufacturer since 2016 | ||||
| chemBlink standard supplier since 2017 | ||||
| Auben Chemical Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 19121901603 | |||
 |
sales@aubenchem.com | |||
| Chemical distributor since 2023 | ||||
| chemBlink standard supplier since 2025 | ||||
| BroadPharm | USA | Inquire | ||
|---|---|---|---|---|
 |
+1 (858) 536-7788 | |||
 |
sales@broadpharm.com | |||
| Chemical manufacturer | ||||
| Classification | Pharmaceutical intermediate >> Heterocyclic compound intermediate >> Pyrimidine compound >> Amine |
|---|---|
| Name | 4-(tert-Butyl)benzene-1,2-diamine |
| Synonyms | 4-(tert-Butyl)-o-phenylenediamine |
| Molecular Structure | 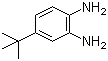 |
| Molecular Formula | C10H16N2 |
| Molecular Weight | 164.25 |
| CAS Registry Number | 68176-57-8 |
| EC Number | 640-307-2 |
| SMILES | CC(C)(C)C1=CC(=C(C=C1)N)N |
| Density | 1.0±0.1 g/cm3 Calc.* |
|---|---|
| Melting point | 98 - 100 ºC (Expl.) |
| Boiling point | 290.5±33.0 ºC 760 mmHg (Calc.)* |
| Flash point | 153.0±24.9 ºC (Calc.)* |
| Index of refraction | 1.575 (Calc.)* |
| * | Calculated using Advanced Chemistry Development (ACD/Labs) Software. |
| Hazard Symbols |

 GHS06;GHS07 Danger Details
GHS06;GHS07 Danger Details | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hazard Statements | H301-H302-H311-H312-H315-H319-H331-H332-H335 Details | ||||||||||||||||||||||||||||||||||||
| Precautionary Statements | P261-P262-P264-P264+P265-P270-P271-P280-P301+P316-P301+P317-P302+P352-P304+P340-P305+P351+P338-P316-P317-P319-P321-P330-P332+P317-P337+P317-P361+P364-P362+P364-P403+P233-P405-P501 Details | ||||||||||||||||||||||||||||||||||||
| Hazard Classification | |||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||
| SDS | Available | ||||||||||||||||||||||||||||||||||||
|
4-(tert-Butyl)benzene-1,2-diamine is an aromatic diamine in which a benzene ring is substituted with amino groups at the 1 and 2 positions (ortho position) and a tert-butyl group at the 4 position. Its molecular formula is C10H16N2. The compound is typically a pale yellow to light brown crystalline solid, moderately soluble in organic solvents such as ethanol, methanol, and dichloromethane, and sparingly soluble in water. The presence of the two amino groups and the bulky tert-butyl substituent influences its reactivity and steric properties in chemical synthesis. The compound is of interest in organic chemistry due to its use as an intermediate for the synthesis of heterocyclic compounds, dyes, and functionalized aromatic molecules. Aromatic ortho-diamines are known for their ability to undergo condensation reactions with carbonyl-containing compounds to form heterocycles such as benzimidazoles and quinoxalines. The tert-butyl substituent provides steric hindrance, which can influence selectivity in reactions and improve the stability of the resulting products. Synthesis of 4-(tert-butyl)benzene-1,2-diamine is commonly achieved through the reduction of the corresponding dinitro compound, 4-(tert-butyl)-1,2-dinitrobenzene, using reducing agents such as iron powder with acid, tin(II) chloride, or catalytic hydrogenation. The dinitro precursor is typically prepared by nitration of 4-tert-butylaniline or by electrophilic aromatic substitution starting from tert-butyl-substituted benzene derivatives. The final diamine is purified by recrystallization from polar solvents or by chromatographic separation to remove unreacted nitro compounds or side products. Chemically, 4-(tert-butyl)benzene-1,2-diamine is reactive due to the nucleophilic amino groups. These amines readily participate in condensation reactions with aldehydes, ketones, and carboxylic acid derivatives to form imines, amides, or heterocycles. The tert-butyl group at the para position stabilizes the aromatic ring and can influence regioselectivity during substitution reactions. The compound also exhibits basicity due to the amino groups and can form salts with acids, which enhances solubility in polar solvents. In practical applications, 4-(tert-butyl)benzene-1,2-diamine is used primarily as a building block for the synthesis of pharmaceuticals, agrochemicals, and dyes. Its ortho-diamine functionality allows for the preparation of benzimidazole derivatives, which are common scaffolds in medicinal chemistry for antimicrobial, antiviral, and anticancer agents. The compound is also used in the production of functionalized aromatic intermediates in organic materials chemistry, such as ligands for metal complexes or polymer precursors. Physically, the compound is stable under ambient conditions but should be protected from strong oxidizing agents, which can convert the amino groups to nitroso or nitro derivatives. Handling precautions include gloves and eye protection, as aromatic diamines can be irritants and may pose health risks upon prolonged exposure. The solid should be stored in a cool, dry environment away from light and moisture. Overall, 4-(tert-butyl)benzene-1,2-diamine is a versatile aromatic diamine intermediate with applications in organic synthesis, medicinal chemistry, and materials science. Its combination of nucleophilic amino groups and sterically bulky tert-butyl substituent enables the preparation of diverse heterocycles, functionalized aromatic molecules, and derivatives with biological or material significance. References 2020. Study of the Effect of Substituents of ortho-Phenylenediamines in the Opening of Lactones and Lactams for Access to Benzimidazol-2-yl Alkanols and Benzimidazol-2-yl Alkylamines. Synlett. DOI: 10.1055/s-0040-1707112 |
| Market Analysis Reports |
| List of Reports Available for 4-(tert-Butyl)benzene-1,2-diamine |