Online Database of Chemicals from Around the World
| Capot Chemical Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (571) 8558-6718 +86 13336195806 | |||
 |
capotchem@gmail.com sales@capotchem.com | |||
 |
QQ chat | |||
| Chemical manufacturer | ||||
| chemBlink standard supplier since 2006 | ||||
| SL Drugs and Pharmaceuticals Pvt. Ltd. | India | Inquire | ||
|---|---|---|---|---|
 |
+91 (40) 6661-1133 | |||
 |
enquiry@sldrugs.com | |||
| Chemical distributor since 1999 | ||||
| chemBlink standard supplier since 2010 | ||||
| Win-Win Chemical Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (577) 6449-8589 +86 15325081899 | |||
 |
sales@win-winchemical.com winwinchemical@gmail.com | |||
 |
Skype Chat | |||
 |
QQ chat | |||
| Chemical manufacturer since 2007 | ||||
| chemBlink standard supplier since 2011 | ||||
| SynQuest Labs, Inc. | USA | Inquire | ||
|---|---|---|---|---|
 |
+1 (386) 462-0788 | |||
 |
Sales@synquestlabs.com | |||
| Chemical manufacturer | ||||
| chemBlink standard supplier since 2011 | ||||
| Alfa Chemistry | USA | Inquire | ||
|---|---|---|---|---|
 |
+1 (201) 478-8534 | |||
 |
inquiry@alfa-chemistry.com | |||
| Chemical distributor since 2012 | ||||
| chemBlink standard supplier since 2012 | ||||
| Hangzhou Leap Chem Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (571) 8771-1850 | |||
 |
market19@leapchem.com | |||
 |
QQ chat | |||
| Chemical manufacturer since 2006 | ||||
| chemBlink standard supplier since 2015 | ||||
| Amadis Chemical Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (571) 8992-5085 | |||
 |
sales@amadischem.com | |||
| Chemical manufacturer since 2010 | ||||
| chemBlink standard supplier since 2015 | ||||
| Shanghai Fuxin Pharmaceutical Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (21) 3130-0828 +86 18645121291 | |||
 |
contact@fuxinpharm.com | |||
| Chemical manufacturer since 2016 | ||||
| chemBlink standard supplier since 2018 | ||||
| Classification | Organic raw materials >> Organic fluorine compound >> Fluoroaniline series |
|---|---|
| Name | 3-Bromo-4,5-difluoroaniline |
| Molecular Structure | 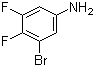 |
| Molecular Formula | C6H4BrF2N |
| Molecular Weight | 208.00 |
| CAS Registry Number | 875664-41-8 |
| EC Number | 642-514-3 |
| SMILES | C1=C(C=C(C(=C1F)F)Br)N |
| Solubility | Very slightly soluble (0.31 g/L) (25 ºC), Calc.* |
|---|---|
| Density | 1.788±0.06 g/cm3 (20 ºC 760 Torr), Calc.* |
| Boiling point | 252.8±35.0 ºC (760 Torr), Calc.* |
| Flash point | 106.7±25.9 ºC, Calc.* |
| * | Calculated using Advanced Chemistry Development (ACD/Labs) Software V11.02 (©1994-2015 ACD/Labs) |
| Hazard Symbols |

 GHS06;GHS07 Danger Details
GHS06;GHS07 Danger Details | ||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hazard Statements | H301+H311+H331-H302-H312-H315-H319-H332-H335 Details | ||||||||||||||||||||||||||||||||
| Precautionary Statements | P261-P262-P264-P264+P265-P270-P271-P280-P301+P316-P301+P317-P302+P352-P304+P340-P305+P351+P338-P316-P317-P319-P321-P330-P332+P317-P337+P317-P361+P364-P362+P364-P403+P233-P405-P501 Details | ||||||||||||||||||||||||||||||||
| Hazard Classification | |||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||
| SDS | Available | ||||||||||||||||||||||||||||||||
|
3-Bromo-4,5-difluoroaniline is a halogenated aromatic amine with the molecular formula C6H4BrF2N. Structurally, it consists of a benzene ring substituted with an amino group (-NH2) at the 1-position, a bromine atom at the 3-position, and fluorine atoms at the 4- and 5-positions. The compound typically appears as a pale yellow to off-white crystalline solid that is moderately soluble in polar organic solvents such as ethanol, methanol, and dichloromethane, but sparingly soluble in water. The electron-withdrawing halogens influence both the reactivity of the amino group and the overall chemical behavior of the aromatic ring. The development of halogenated anilines like 3-bromo-4,5-difluoroaniline is largely motivated by their utility as intermediates in pharmaceutical, agrochemical, and material chemistry. The presence of electron-withdrawing halogens can enhance metabolic stability, modulate electronic properties, and improve binding affinity in medicinal chemistry applications. Such substituted anilines are commonly used to construct heterocyclic compounds, dyes, and ligands for metal-catalyzed reactions. Synthesis of 3-bromo-4,5-difluoroaniline generally involves selective halogenation of a suitably protected aniline derivative. One approach begins with 4,5-difluoroaniline, followed by electrophilic bromination at the 3-position under controlled conditions using a brominating agent such as N-bromosuccinimide or bromine in acidic or organic solvent. Reaction parameters, including temperature, solvent choice, and stoichiometry, are carefully optimized to favor mono-bromination at the desired position and to avoid overbromination or substitution at unintended sites. The product is purified by recrystallization or chromatographic techniques to yield a stable, high-purity compound. Chemically, 3-bromo-4,5-difluoroaniline exhibits typical aniline reactivity, including nucleophilic substitution and condensation reactions. The amino group can participate in acylation, sulfonation, or diazotization reactions, while the halogen substituents influence regioselectivity and electronic characteristics. The combination of bromine and fluorine atoms provides synthetic versatility, as the bromine atom can undergo cross-coupling reactions such as Suzuki, Heck, or Sonogashira coupling to build more complex aromatic or heteroaromatic frameworks. In practical applications, 3-bromo-4,5-difluoroaniline is used primarily as an intermediate in the synthesis of pharmaceuticals, agrochemicals, and functional organic materials. Its halogenated aromatic structure enables the preparation of biologically active compounds, fluorescent dyes, and polymer precursors. In medicinal chemistry, the compound serves as a key building block for creating molecules with optimized lipophilicity, metabolic stability, and receptor binding. In materials science, its incorporation into aromatic frameworks can modulate electronic and optical properties. Physically, the compound is stable under standard storage conditions but should be protected from strong oxidizing agents and moisture, which can degrade the amino group or halogen substituents. Handling precautions include gloves, eye protection, and adequate ventilation to prevent irritation from dust or vapors. It should be stored in a cool, dry place in tightly sealed containers. Overall, 3-bromo-4,5-difluoroaniline is a versatile halogenated aniline intermediate with significant utility in organic synthesis, medicinal chemistry, and materials research. Its combination of amino functionality and selective halogenation provides chemical reactivity, synthetic flexibility, and potential for incorporation into biologically and functionally important compounds. References 2024. 6-aza-quinoline derivatives and related uses. WO Patent. URL: WO-2023039505-A1 2024. GCN2 and perk kinase inhibitors and methods of use thereof. US Patent. URL: US-11912668-B2 |
| Market Analysis Reports |
| List of Reports Available for 3-Bromo-4,5-difluoroaniline |